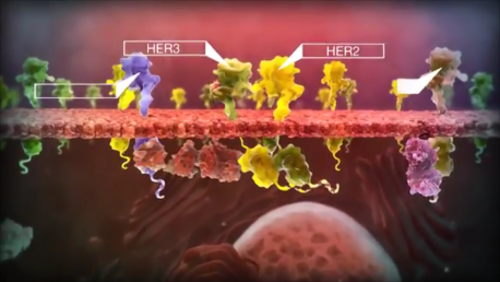
Aequilibrium pharma is a privately owned, fully integrated specialty biopharmaceutical company focused exclusively on development and manufacturing of high quality biosimilar products. We are specialists in biogeneric product creation, all along the value chain from cell line development to commercial manufacturing.
Aequilibrium Pharmaceutical company are continuously searching for the safest, fastest and most cost effective methods to bring quality drugs to market. Biosimilars, Biotech, Pharmaceuticals, generics products are developed under tight deadlines, competitive pressures and regulatory compliance.
Aequilibrium Pharmaceuticals work to ensure consistent revenue streams, speed production and delivery of products, minimize disruption and adhere to FDA& EMA guidance, compliance & regulatory requirements such as Current Good Manufacturing Processes (CGMP), (GxP) and 21CFR11.
Aequilibrium Pharmaceuticals multimedia management systems solutions require delivery of a unified, configurable, multimedia control platform for marketing, R&D, clinical systems, health data, as well as trial outcomes and health research to enable researchers to gain insights into the viability, safety, and efficacy of new and existing therapies. Equilibrium can help drive greater discovery productivity and regulatory compliance through its extensive product line.